|
What happens if you chew gum and chocolate at the same time? You'll find out in this sweet candy science experiment while learning an important concept in chemistry: solubility. Click and expand the tabs below to get started experimental procedure
what's happening
This is a chemistry experiment, even though you are doing it in your mouth! It illustrates a very important concept in chemistry called solubility, which is the ability of one substance to dissolve another substance. Chemists say that “like dissolves like” and this is an example of that. If your gum was candy coated, like a Chiclet or gum-ball, you should have noticed that the candy coating dissolved or disappeared as soon as you began chewing. This is because the candy coating was made of sugar, which easily dissolves in water (try adding a spoon full of table sugar to a glass of water and watch what happens), and the saliva in your mouth is mostly water. Chemists would say that sugar is soluble in water. Saliva and chewing are the first steps in digesting or breaking down your food into simpler components that your body can use for energy. Your saliva does not dissolve the gum, however, because most modern chewing gums are made of a synthetic rubber (i.e. man-made, not natural), which is a type of oil-based polymer, such as butadiene-styrene, vinyl acetate, or polyethylene (the same material in plastic grocery bags). You may have already learned that oil (as well as oil-based substances) and water don't mix- they are not alike. But chocolate does contain oil-based fatty substances, so the chocolate dissolves the gum. Like dissolves like. After adding the first piece of chocolate and chewing you should have noticed that the gum was much softer and stickier, and maybe much smaller than it was after you first chewed the gum alone. If you add enough chocolate to the gum in your mouth (you may need 3 or 4 pieces) and continue chewing you should be able to make the gum disappear completely! variations and related activities
If you're not allergic to peanuts, try chewing a spoon full of peanut butter with your gum instead of chocolate. Does the peanut butter also make the gum disappear? Why do you think that is? Instead of doing the entire experiment in your mouth, just chew the gum for a minute or two in your mouth, then put it in a cup or small bowl instead. Add a couple spoonfuls of vegetable oil to the cup and use a spoon to stir and grind the gum. Does it dissolve? If not, add a little more vegetable oil. Try repeating ths experiment with some other liquids. Is gum more or less soluble in other liquids? Solubility has it's limits, however, and you can easily test this. Add one or two spoonfuls of sugar to a glass of water and observe what happens. Does all of the sugar dissolve immediately? Observe for a minute or two, has more dissolved? Use a spoon tp stir the water and sugar. Does it dissolve more easily? Try adding more spoonfuls of sugar and stirring. Does the sugar continue to dissolve? Keep adding sugar until it will no longer dissolve in the water. Now you have reached the solubility limit- the water simply can't hold any more sugar and we say it is saturated. What would happen if you added more water to the glass? Solubility also depends temperature. With the help of an adult try repeating this experiment with warm or hot water. Is sugar more soluble in hot water, i.e. can you make more spoonfuls of sugar dissolve? Coming soon: make rock candy float letters off M&M's pop rocks dancing raisins carbonated soft drinks Lava Lamps Dissolve egg shell in vinegar Underwater fireworks (food coloring dissolves in water but not oil) Oil-based and water-based paints references and links to more information
Journal of Chemistry Education Classroom Activity #105. A Sticky Situation: Chewing Gum and Solubility Learn more about solubility: Coming soon: Saliva Digestion Solubility experiments
Return to Try Science at Home
1 Comment
Click and expand the tabs below to get started. experimental procedure
what's happening
Modern disposable baby diapers contain a small amount (4-5 grams) of polyacrylic acid in a powder form (sodium polyacrylate), which is mixed into the fluff in the middle layer of the diaper. The inside layer of the diaper allows water to pass through it into the absorbent middle layer, and the outer layer is waterproof, so both baby and mommy stay dry. Polyacrylic acid can absorb about more than 100 times its own weight in water, which is why an average sized diaper can absorb up to 16 ounces of water. Luckily that's more than a baby usually pees.
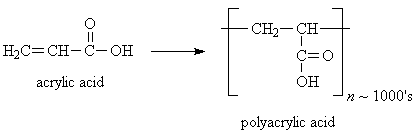
Why is this powder so good at absorbing water? Polyacrylic acid is a polymer made from the monomer acrylic acid. Polymer molecules are kind of like long chain made up of lots of identical links (the monomers). Polyacrylic acid chains contain thousands of monomer units, as well as cross-linking between different molecule chains, which makes them kind of tangle together. Most polymers, such as polyethylene and polystyrene (used in trash bags, plastic bottles, and Styrofoam®, for example) are hydrophobic, meaning they repel water. Polyacrylic acid, however, is very hydrophilic - it attracts water- because of the carboxylic acid groups (COOH) in the polymer, which can hydrogen-bond to water molecules. The cross-linking also helps trap lots of bonded water molecules inside the tangled chains.
Variations and related activities
You can make this activity more colorful by adding a few drops of food coloring to the water. Also try adding some table salt to the water. What happens? [Table salt is sodium chloride (NaCl) and the chloride ions will bond with the SAP and prevent water molecules from doing so.] If you place the wet polymer on a towel or napkin and allow it to dry out for a few days it will dehydrate and shrink back to almost its original powder form and can be used over an over again. Polyacrylic acid, often just called baby diaper polymer or super absorbent polymer (SAP), comes in many forms and has many other applications. SAP is often used to clean up hazardous waste spills- even more hazardous than what ends up in a baby diaper! Just sprinkle the dry powder on the spilled liquid and then sweep or vacuum it up. You can also find SAP in moisture-control potting soils at a garden store. If you over-water your plants the SAP will absorb the excess water. As the soil dries the SAP dehydrates and the excess water returns to the soil, maintaining just the right amount or moisture for the plant to thrive. You can also buy headbands and neckbands that contain SAP inside, after you wet them the water evaporates slowly and keeps you cool for a long time (see the reference link below to make your own!) You can buy Instant Snow powder from many stores online (see references below). Instant snow is exactly the same material, it's just ground up into a much finer powder. This causes it to form smaller molecular structures and puff up instantly when it absorbs water, giving it the look and feel of real snow. It's often used in movies when they want snow in a scene without the need for cold temperatures- and the wet mess when it melts! Orbeez and other water beads are clear spheres and other fun shapes made from the same polyacrylic acid SAP material, but prepared a little differently to form the shapes you see when they absorb water. Typical Orbeez spheres start as 3 or 4 mm beads, but can grow to more than 1" in diameter as they absorb water. Because they are composed almost entirely of water when fully grown, they are essentially invisible in a bowl of water (as long as both the spheres and the liquid water are clean). Water is water. The index of refraction (a measure of how light bends as it passes through a material) of the liquid water is the same as the water in the sphere, so light rays are not refracted as they pass from the water in the bowl to the water in the beads, and the spheres are therefore almost completely indistinguishable from the rest of the water. They also bounce (but can break if you bounce them too hard) and act as little magnifying glasses. Just as with the baby diaper polymer, you can add food colors to make colored Orbeez, and if you let them dry they will shrink back down to little beads that you can use over and over again. references and links to more information
The science of sodium polyacrylate and SAP's:
Here are some other ways to do this experiment:
This video from Science Beyond at the St. Louis Science Center shows you the secret behind one of our most popular demonstrations: Fun with Instant Snow powder: Orbeez!: Make your own cooling headband or neck bandana with SAP:
Return to Main Menu
|