 You won't believe how easy it is to make your own butter simply by shaking a cup of heavy whipping cream, which comes from milk. Give it a try- and you even get to eat this experiment when you're done! Click tabs below to get started what you'll need
For whipped cream (optional):
Be sure to ask your mom, dad or another adult to help-you can share your butter with them. experimental procedure
Once you finish rinsing your butter, remove all the water and taste it on some bread or a cracker. Most butter you find in the grocery store has salt added, but your butter is unsalted (sometimes called sweet butter). If you don't like the taste, add a pinch of salt. Additional Optional Experimental Procedure: You can also make your own real whipped cream.
what's happening
Butter is very interesting scientifically. We used cream to make butter, but where does cream come from? Cream comes from cows. It is part of the milk that a cow gives. It has a lot of fat in it. Fat occurs in pieces called molecules. These molecules like to stick together in big clumps, but at the dairy they homogenized the milk to keep the fat and everything else all mixed up well. When you shake the cream, however, the molecules which were floating around start sticking to each other. When enough of them stick together they won't float or stay mixed up any more and you have butter!
more detailed explanation
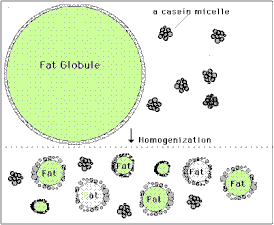
You've probably heard that oil and water don't mix, but milk is in fact a mixture of many different components, including sugars, proteins, fats and yes, water. [Fats and oils are basically similar molecules, also often called lipids, and made of smaller building blocks called fatty acids. Although fat, oil and lipid are often used to refer to fats, "oil" generally refers to a fat that is liquid at normal room temperature and "fat" is solid at room temperature.] The oil or fat in milk can mix with water without separating because they form into little globules sort of like water balloons. The fat is inside a thin skin or membrane which helps attract water somewhat, and because fat is less dense than water, the globules float and form what's called a colloidal suspension or emulsion. The smaller the globules, the easier it is for them to remain emulsified, but if they are too large they will separate and float to the surface. Years ago before milk was homogenized, the fat globules would float to the top and form a layer called the cream, which contained most of the milk's fat. Homogenization breaks up the larger globules into smaller ones, which, along with the milk proteins that then help form a stronger globule membrane, prevent this separation. The cream you buy at the grocery store is separated at the dairy and then homogenized to keep the fat suspended in the liquid. Heavy whipping cream means that it has a very high fat content, 36-40% (normal cream is about 30% fat, while Half & Half is 12% and whole milk 3.5% fat). Butter is essentially pure milk fat.
In our heavy cream, the fat globules are still emulsified, or suspended in the water along with some protein and sugar. If we shake the cream though, the globules will begin to crash into one another and pop or burst their membranes. When this happens the fat molecules spill out and clump together with other fat molecules. At first as this happens the fat traps a lot of air and forms bubbles or foam, which is the whipped cream. The more you shake it, however, the more bubbles pop and the more densely the fat clumps become until they can no longer remain suspended in the water, and they separate completely. This is the butter, and the remaining liquid, mostly water and proteins, is called buttermilk. The butter you make is not only good to eat, but will stay fresh for a long time, especially if it is thoroughly rinsed of all the milk liquid, since the milk can spoil. Adding sugar to the whipped cream not only sweetens and thickens it, but helps prevent the fat bubbles from popping so that it will stay a light fluffy foam much longer. variations and related activities
Try using cold heavy whipping cream to make butter. Try warm cream. Does it take more or less shaking to get to butter? How do you think temperature affects this process? If you like these milk science activities, you can also try our Milk Fireworks experiment, as well as making cottage cheese and glue (see link below) from milk. references and links to more information
Others versions of this activity:
Experiment with the temperature of the cream: Learn more about milk chemistry: What is homogenized milk?: Whipped cream as a science fair project:
What happens when whipped cream comes out of a can?: You can also make glue from milk:
Return to Main Menu
1 Comment
Basic Procedure:
If everything works, you should see the food coloring begin moving around, leaving colored streaks and swirls on the milk surface. You may also see bursts of color suddenly appear from an otherwise plain white surface, just like colorful volcanoes or fireworks erupting. If nothing happens after several seconds, try touching another drop of soap to the milk surface, perhaps in a different spot. You can also try gently rubbing a little soap on the bottom of the bowl, or even carefully adding a drop of soap directly to the surface, but don't add too much soap. A drop or two is all you should need. Be patient- sometimes nothing may seem to be happening for a minute or more, but colors are moving around below the surface and will suddenly burst into view. Remember not to stir the milk or shake the bowl- we want the colors to move on their own, not because you move them! Additional Experiments:
What's Happening: Mother's milk is an amazing substance, which really isn't surprising considering that it contains all of the nutrients a baby needs to survive and grow. The plain white liquid you see is actually a uniform (homogenous) mixture consisting of fats or oils (composed of molecules called fatty acids), sugars, proteins, vitamins and some other chemicals, but mostly water molecules. This is called an emulsion or suspension- a mixture of liquids and solids, most of which are not truly dissolved. Normally these various components would separate (and in raw or non-homogenized milk some, notably the fats, still do), but the fatty acid and protein molecules have both hydrophilic (water-loving) and hydrophobic (water-hating) regions, which causes these long molecules to twist around and group together into more stable arrangements, making it possible for them to stay suspended in the water. The fatty acids form globules, sort of like little water balloons (or in this case, fat balloons), with their hydrophobic ends on the inside and their hydrophilic ends on the outside facing the water molecules. The proteins form similar balloon-like structures called micelles. Thus, the milk is in a complex state of equilibrium (i.e. not changing), with all the molecules not exactly "happy" to be where they are, but at least willing to stay put as long as they aren't "upset" too much. When you gently add the food color (which are dissolved in water) they are also happy to stay put in their own tiny puddles. Molecules of dish detergent, like fats and protein, also have both hydrophilic and hydrophobic regions, so adding them to the surface of the milk completely disrupts the existing state of equilibrium as the soap molecules grab and pull on the fat globules and protein micelles. Scientists say that on a molecular level, this soapy region of the milk is in a state of chaotic disarray and all of the various molecules begin to move about, dragging the food colors with them as they try to return to some equilibrium. In other words, before the soap was added the molecules in the milk were at least where they liked to be and didn't want to be disturbed. Then come along these annoying soap molecules like bullies that want to disturb everything (on a molecular level, of course). Because milk is all white, we wouldn’t normally be able to see what's happening, but as the food coloring is sort of caught in the middle we can observe all the action taking place. The fats in milk are largely responsible for the action in this experiment, so milks with more or less fat may behave differently, including milks from other mammals (see the link below). Temperature also affects many physical and chemical reactions because more energy is available at higher temperature. References and links to more information: Others versions of this activity:
Milk from other mammals: What is homogenized milk?: Learn (LOTS) more about milk chemistry:
Experimental Procedure:
What’s Happening: The science behind this is actually rather complex, and is explained in much greater detail in our Instant Freeze Super-Cooled Water lab. Basically, when salt (or anything else) is dissolved in water it lowers the freezing point of the salt-water solution below that of pure water alone. In other words, when you put the salt and the ice together, you are making a solution that can get much colder than 32 degrees Fahrenheit (the normal freezing or melting temperature of pure water) and still remain liquid. This is called freezing point depression, and it can be very useful when you are trying to get something else really cold (in this case, the ice cream). Freezing point depression is also why the milk or cream needs to be much colder than 32°F before it will freeze; milk is mostly made of water, but with a lot of other stuff dissolved in it, and that lowers its freezing point too. As you shake the big bag, sloshing around the smaller bag inside, the super cold salt-ice-water solution in the big bag takes heat away from the cream/milk inside the smaller bag, lowering the temperature enough for it to freeze. You should also notice that this heat flowing out of the small bag melts even more of the ice in the big bag- it gets sloshier as you shake it. Of course heat from your hands does the same thing (and you might want to wear gloves as you shake the bags). It's very important that you shake the bags vigorously while the milk/cream is freezing. This breaks up the ice crystals that are forming inside the smaller bag and keeps your final ice cream smooth and creamy- just the way you probably like it. If you don't shake the bags, as these ice crystals form they will stick to each other and you'll end up with a hard frozen block of milk instead- a milk-sicle! Try it by placing another Zip-Lock bag or cup of milk in your freezer to see what happens. Variations and Related Activities: Here's another way to make ice cream, which uses the same ideas, but slightly different materials:
You can play around with many different ways to make ice cream. What if you used skim milk? 2% milk? A combination of the two? Does it take longer for them to freeze? What if you used chocolate milk? Do you prefer richer ice cream (made with pure cream), or not-so-rich ice cream (made with Half and Half)? What about ice milk (which is ice cream made with milk and no cream at all)? Which tastes the best? Another way to do this is to get two metal cans (coffee cans work well). One should be able to fit inside the other, with room for the ice and salt. It should also have a very tight-fitting lid. Put the milk/cream mix inside the small can, and put the lid on it. You might want to use duct tape to make sure that the lid stays on. Then, put the small can inside the big can. Put the ice, salt and a little liquid water inside the big can, around the small can. Put the lid on the big can, and duct tape it shut. Then, play soccer with the can for about 15-20 minutes! Untape the cans, wipe off the little can, and enjoy the ice cream! Links to more information and activities: More versions of this activity:
The science of ice cream: More homemade ice cream recipes and techniques:
Turn this activity into a real experiment:
What you'll need:
As always, be sure to ask your mom, dad or another adult to help- they'll want to taste your result. All utensils and ingredients should be clean and food-safe if you want to taste your results. Experimental Procedure:
What's Happening: Mother's milk is an amazing substance, which really isn't surprising considering that it contains all of the nutrients a baby needs to survive and grow. The plain white liquid you see is actually a uniform (homogenous) mixture consisting of fats or oils (composed of molecules called fatty acids), sugars, proteins, vitamins and some other chemicals, but mostly water molecules. This is called an emulsion or suspension- a mixture of liquids and solids, most of which are not truly dissolved. Normally these various components would separate (and in raw or non-homogenized milk some, notably the fats, still do), but the protein and fat molecules have both hydrophilic (water-loving) and hydrophobic (water-hating) regions, which causes these long molecules to twist around and group together into more stable arrangements, making it possible for them to stay suspended in the water that makes up most of milk by volume. The proteins form micelles, sort of like little water balloons (or in this case, protein balloons), with their hydrophobic ends on the inside and their hydrophilic ends on the outside facing the water molecules. The fat molecules form similar but much larger balloon-like structures called globules. Our "I can't believe It IS Butter!" experiment dealt with the fat globules in milk, while in this experiment we focus instead on the protein micelles. There are dozens of different proteins in milk, but they can be grouped into two main categories: the so-called curds and whey proteins from the "Little Miss Muffet" nursery rhyme. In cow's milk the curd proteins, called caseins, outnumber whey proteins about 4 to 1 by weight. One important difference is that caseins will coagulate or form solid clumps under acidic conditions while the whey proteins will remain suspended in the liquid, and that is what is happening in this experiment. Milk solid curds are used to make cheeses, or in our case cottage cheese. Most cottage cheese you buy in the grocery store is salted, which is why the cottage cheese curds you made may have tasted a little different (you may have also tasted the lemon or lime if you didn't rinse them well). Lemons and limes have an acid called citric acid in their juice, as do other citrus fruits like oranges and grapefruit. Normally the casein micelles have negative electric charge on their outer surfaces so that they all repel each other and remain suspended in milk. When you added lemon or lime juice to your milk the citric acid neutralized these negative charges and allowed micelles to begin sticking together in larger and larger clumps, which also trap much of the fat in the milk as they form. We can also say that the milk has curdled. It was these casein curds that you caught in your strainer, while the liquid that passed through contained the remaining whey proteins. Heating the milk also strongly enhances this coagulation process. Special chemicals called enzymes can also be used to produce milk curds (described in some of the links below). In this experiment you produced a simple cottage cheese, which is essentially the first step in making any kind of cheese, though most types require many more steps (see links below for more on cheese making). Sometimes when milk spoils it can curdle (see link below) due to bacteria converting the primary sugar found in milk- lactose- into lactic acid which causes casein to coagulate just as you did with citric acid. The process of converting sugar to acid with bacteria is a type of fermentation, and this is how yogurt is made (although the fermentation is carefully controlled so the milk doesn't actually spoil). Variations and Related Activities: Try other citrus fruits in place of the lemon or lime, or different acids such as vinegar (acetic acid). Another interesting experiment uses fresh pineapple juice, which is too weak an acid to be very effective but also contains an enzyme that can produce curds by a different mechanism (see the Scientific American version below). You can also try different milks (low fat, skim, buttermilk, etc.) A fun extension of this experiment is to make your own casein glue. For our cottage cheese we used acid to make the casein micelles coagulate, which allowed us to separate them from the other whey proteins. The opposite of an acid is a base, and if we add a base like baking soda (sodium bicarbonate) to the casein curds we can reverse the process and re-suspend the sticky casein molecules in liquid to make a simple type of glue (links below). References and links to more information: Others versions of this activity:
Make better cottage cheese: Cheese making:
Why milk spoils: Milk Science Classroom Activities for older students:
Learn more about milk chemistry:
Making Casein Glue:
|