|
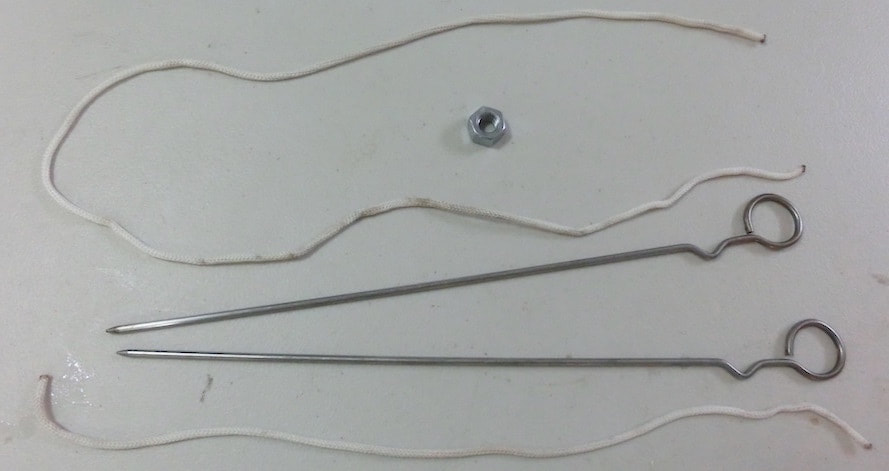
Click and expand the tabs below to get started what you'll need
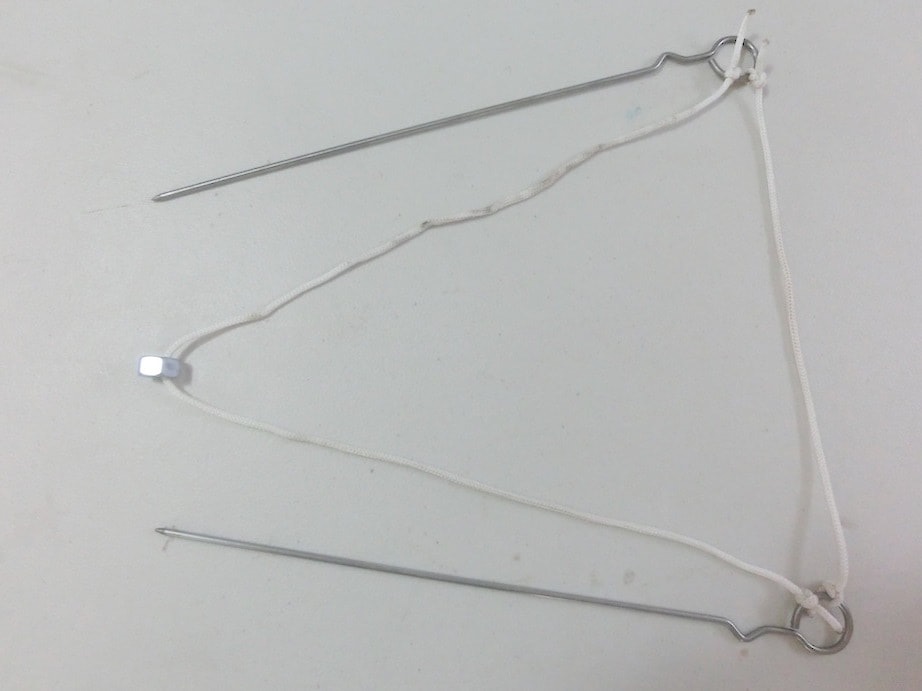
basic procedure
making giant bubbles
what's happening
Did you know that water molecules are actually very sticky? You've probably seen them clinging to lots of surfaces like walls, windows and tables. When one material sticks to another we call it adhesion, and water molecules adhere or stick to many other materials, but they also like to stick to other water molecules. When a material sticks to itself we call it cohesion. If there is no good surface around for water to stick to it's perfectly happy sticking to itself by pulling on several other water molecules nearby. In fact, each water molecule pulls so hard on the others around it that all of the water tries to pull itself into one tight spherical ball if it can't find anything else easier to stick to. This force is sometimes called surface tension. Pour some water onto a freshly waxed car or tabletop and it forms lots of little droplets or "beads" because it can't stick as well to the waxy surface (check the video link at the end to see what water does on the International Space Station). If you pour it on most other surfaces, however, it will spread out if it can stick to those surfaces. Okay, so what's all this have to do with soap bubbles? A bubble is like a balloon, with a thin film or skin on the outside surrounding and trapping air (or some other gas) inside. Water doesn't stick to air though, leaving only other water molecules for it to stick to, but the surface tension of water, or the force with which it pulls on other nearby water molecules, is so strong that it will form small beads full of water (like raindrops) rather than stretching into a thin film. If we want to stretch water into a thin skin to make a bubble we need to add something else that it would rather stick to, and that's exactly what soap does. Soaps and detergents are usually long snake-like molecules with one end (let's call it the head) that likes to grab or pull on water molecules while the other end (tail) likes to get as far away from water as possible (or if possible grab something very different like oil or grease, which, by the way, is what makes them so good at cleaning dirt). The head ends of soap molecules in your bubble solution grab onto water molecules, but since their tail ends want to get out of the water at the same time, they can only grab one side so that their tail ends can remain in the air. Other soap molecules can also grab onto the same water molecules but from the opposite side, while also keeping their tail ends in the air. This leaves the water molecules free to grab some other water molecules, but only in a region where there is no soap, forming a thin three-layer film skin. You can think of these three layers sort of like a peanut butter sandwich, where the pieces of bread on the top and bottom are the soap layers and the peanut butter inside is the water. The surface tension of the water layer inside gives the skin of your bubble its strength, while the soap layers above and below weaken the surface tension just enough to allow the water to stretch out into a thin film so that you can blow a bubble. Materials like soap that reduce the surface tension of water (or other liquids) in this way are called surfactants. The soap-water-soap film may be strong enough to form a skin of a bubble but it's also very, very thin- thinner than the hair on your head- so there is a limit to how big a bubble you can make with just the cohesive force of water holding it together. Making it any bigger and the skin will break, popping the bubble. The guar gum (or cornstarch) you added to the water gives it extra strength to make even bigger bubbles. The baking powder also helps by controlling pH or acidity of the solution. You may have seen some soap bubble recipes that add glycerine to the soap, which works by slowing the evaporation of water (although most bubble experts agree that recipes like ours work much better). Reference links at the end explain in more detail how these ingredients work. variations and related activities
Try blowing a small bubble inside one of your big bubbles. To do this get as close as you can to the big bubble without touching it, then gently blow a puff of air into the wall of the bubble (see the video). Why do bubbles pop? Remember that the skin of a soap bubble is very thin, and it's only the water layer which really holds the bubble together as the soap layers on either side actually weaken the water's cohesive forces or surface tension. As the layer of water evaporates the skin of the bubble gets thinner and thinner until the water molecules can no longer hold onto each other and the bubble pops. Do you think your bubbles would last longer on a hot and dry day, or a cool and humid day? Of course a bubble also pops when you touch it- that is if you touch it with your dry finger or something else that draws the water out of its film just like evaporation does. If you dip your finger or hand into the soap solution you should be able to carefully push it through the skin of the bubble and pull it back out without popping it. You can even catch and hold a bubble in your hand (as long as your hand is completely wet- touch it with any dry skin and it will pop. How can a triangle or square shaped bubble wand still make spherical (ball-shaped) bubbles? Once you release your bubble from the wand you may have noticed that it quickly formed a more spherical shape. This is because a sphere is the most stable shape for a bubble, allowing it to enclose the most air while using the least amount of soap (mathematicians might say enclosing the maximum volume with the minimum surface area). Huge bubbles have a large amount of water in their skins, however, and as gravity pulls on this water (as well as any wind blowing on the bubble) the relatively heavy skin gets tugged quite a bit, which is why the bubble wobbles and changes shape a bit as it moves, but it still tries to remain mostly spherical. Did you also notice how colorful your bubbles are (look at the photo above)? In fact, the different colors form rainbow-like stripes that circle around the bubble (although the colors here are formed in a different way than they are in a rainbow). These colored stripes are called interference fringes and caused by light rays reflecting off the thin soap-water-soap film of the bubble. Some light rays reflect off the outside surface of the film and back to your eyes while others pass through this surface, into the film itself, and then reflect off the inside surface and then back to your eyes. When these various light rays recombine on the way to your eyes we say that they interfere with each other. This also happens when light rays strike a pane of window glass, but the soap film of the bubble is much, much thinner. In fact, the thickness of the soap film is close to the wavelengths of light, so that this interference creates colored bands as the thickness of the film changes, which is why the colors change as the bubble slowly evaporates and its skin gets thinner (check out the references at the end to learn more about the physics of light). Gravity also pulls the water in the bubble downward, so that it's skin is thicker near the bottom. Thus the fringes or bands of color indicate changes in film thickness similar to how the lines on a contour map indicate elevation changes. references and links to more information
Just about everything you ever wanted to know about soap bubbles:
How does soap work, and what is the difference between soap and detergent?:
Experiments and other activities with surface tension and soap:
Surface tension of water in space: Why are bubbles round?:
Colors in soap bubbles (and rainbows, just for fun):
2 Comments
What you'll need
experimental procedure
what's happening
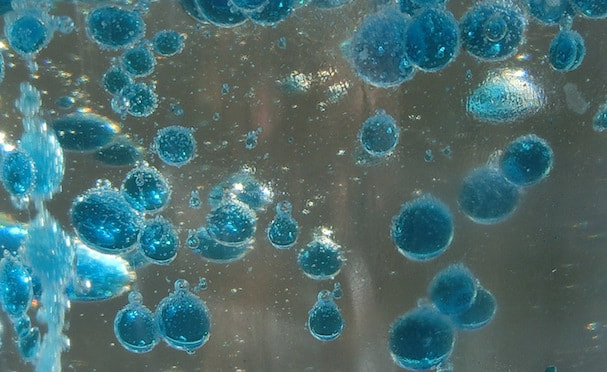
You may have heard someone say that water and oil don't mix. The molecules (the arrangement of atoms that make up these chemicals) that make up water are very different from molecules that make chemicals like vegetable oil. Water molecules (or H2O) attract other water molecules and oil molecules can (weakly) attract other oil molecules, but water molecules strongly repel oil molecules, kind of like the way magnets repel each other if you point the north pole of one towards the south pole of the other. Thus when you add oil to water they will separate into different layers, and the oil layer will float to the surface because oil is less dense than water. This is the scientific term which means that a cup of water has more mass (and is therefore heavier) than the same cup filled with oil. The force that causes the oil to float above the water layer is called the buoyant force or just buoyancy. Since the food coloring is dissolved in water, these drops also will not mix with oil and sink to the bottom of your cup where they burst and mix with the water to color it. The solid Alka-Seltzer pieces are more dense than the oil or the water, so they sink all the way to the bottom. As soon as they reach the water they begin to dissolve and a chemical reaction takes place between two of the ingredients that produces carbon dioxide gas bubbles. As these bubbles rise they pull some of the water along with them and have enough buoyancy to rise through the oil layer, but once they reach the surface the bubbles pop and the gas escapes. Now the water droplets are too heavy to float, and they fall back through the oil where they may pick up another gas bubble to rise again. This process resembles the rising and falling bubbles in a Lava Lamp, but it works a little differently. A real Lava Lamp also contains two immiscible liquids (i.e. they don't mix) with different densities, but it uses the energy from a light bulb underneath to warm the lower liquid. As it warms it expands and its density decreases, allowing bubbles to rise up through the upper liquid. As these bubbles float higher (and farther away from the heat source) they cool down, become denser once again, and fall back into the lower layer where the process can repeat. The upper liquid also warms during this process, but it was carefully chosen so that its density does not decrease as much, so that it still provides enough buoyant force to push the bubbles from the lower liquid upwards. See the reference link below for a more detailed explanation. variations and related activities
references and links to more information
Alternative Lava Lamp experiments:
How real Lava Lamps work: More about the differences between and oil and water molecules:
Try some cool density column experiments:
More about density and buoyancy:
More cool buoyancy experiments:
|