|
What you'll need
experimental procedure
what's happening
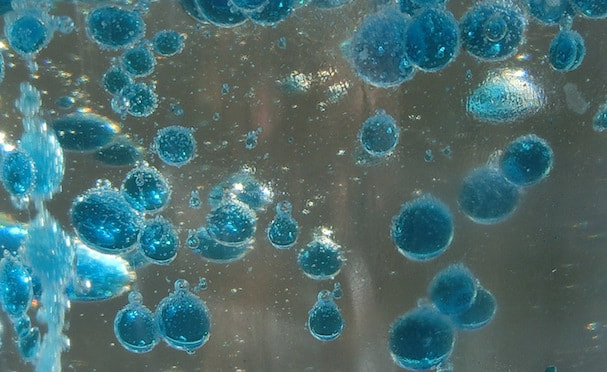
You may have heard someone say that water and oil don't mix. The molecules (the arrangement of atoms that make up these chemicals) that make up water are very different from molecules that make chemicals like vegetable oil. Water molecules (or H2O) attract other water molecules and oil molecules can (weakly) attract other oil molecules, but water molecules strongly repel oil molecules, kind of like the way magnets repel each other if you point the north pole of one towards the south pole of the other. Thus when you add oil to water they will separate into different layers, and the oil layer will float to the surface because oil is less dense than water. This is the scientific term which means that a cup of water has more mass (and is therefore heavier) than the same cup filled with oil. The force that causes the oil to float above the water layer is called the buoyant force or just buoyancy. Since the food coloring is dissolved in water, these drops also will not mix with oil and sink to the bottom of your cup where they burst and mix with the water to color it. The solid Alka-Seltzer pieces are more dense than the oil or the water, so they sink all the way to the bottom. As soon as they reach the water they begin to dissolve and a chemical reaction takes place between two of the ingredients that produces carbon dioxide gas bubbles. As these bubbles rise they pull some of the water along with them and have enough buoyancy to rise through the oil layer, but once they reach the surface the bubbles pop and the gas escapes. Now the water droplets are too heavy to float, and they fall back through the oil where they may pick up another gas bubble to rise again. This process resembles the rising and falling bubbles in a Lava Lamp, but it works a little differently. A real Lava Lamp also contains two immiscible liquids (i.e. they don't mix) with different densities, but it uses the energy from a light bulb underneath to warm the lower liquid. As it warms it expands and its density decreases, allowing bubbles to rise up through the upper liquid. As these bubbles float higher (and farther away from the heat source) they cool down, become denser once again, and fall back into the lower layer where the process can repeat. The upper liquid also warms during this process, but it was carefully chosen so that its density does not decrease as much, so that it still provides enough buoyant force to push the bubbles from the lower liquid upwards. See the reference link below for a more detailed explanation. variations and related activities
references and links to more information
Alternative Lava Lamp experiments:
How real Lava Lamps work: More about the differences between and oil and water molecules:
Try some cool density column experiments:
More about density and buoyancy:
More cool buoyancy experiments:
1 Comment
10/4/2022 03:49:57 am
I can't wait to do this experiment. Thanks for sharing the instructions in detail. Let's have a try whether these instructions will work or not.
Reply
Your comment will be posted after it is approved.
Leave a Reply. |